Spiriva
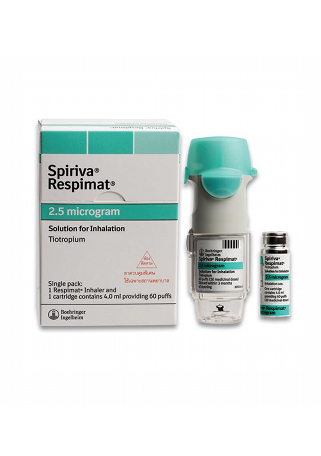
Spiriva
- In our pharmacy, you can buy Spiriva with a prescription only. It is available globally including the US and EU.
- Spiriva is used for the long-term management of chronic obstructive pulmonary disease (COPD) and asthma. It acts as an anticholinergic bronchodilator, specifically a selective muscarinic antagonist.
- The usual dosage for Spiriva HandiHaler is 18 mcg via inhalation once daily. Spiriva Respimat is available in two doses: 2.5 mcg (2 puffs) once daily for COPD and 1.25 mcg (2 puffs) once daily for asthma.
- The form of administration for Spiriva is capsules (HandiHaler) for COPD and an inhalation solution (Respimat) for both COPD and asthma.
- The effect of Spiriva begins within minutes, typically around 30 minutes after inhalation.
- The duration of action is up to 24 hours.
- Do not consume alcohol while using Spiriva.
- The most common side effect of Spiriva is dry mouth.
- Would you like to try Spiriva without a prescription?
Spiriva
Basic Spiriva Information
- INN (International Nonproprietary Name): Tiotropium bromide
- Brand names available in Canada: Spiriva HandiHaler, Spiriva Respimat
- ATC Code: R03BB04
- Forms & dosages: Inhalation powders (capsules), inhalation solutions
- Manufacturers in Canada: Boehringer Ingelheim
- Registration status in Canada: Approved
- OTC / Rx classification: Prescription-only medication
Overview of Spiriva and Its Components
The International Nonproprietary Name (INN) of Spiriva is Tiotropium bromide, a medication designed for individuals with chronic lung conditions. In Canada, Spiriva is marketed under two key brand names: Spiriva HandiHaler and Spiriva Respimat. These inhalation products are essential in treating respiratory diseases, specifically chronic obstructive pulmonary disease (COPD) and for maintaining asthma control.
Classification and Manufacturer Information
Spiriva is classified under the ATC Code R03BB04, indicating its role as an anticholinergic bronchodilator. Primarily, it is administered in two forms for patients: inhalation powders through capsules and inhalation solutions through devices. The main manufacturer behind Spiriva is Boehringer Ingelheim, a recognized entity in the pharmaceutical industry for its commitment to respiratory health solutions.
Regulatory Approval and Medication Classification
Spiriva has received approval from both Canadian health authorities and international organizations, including the FDA and EMA. It is crucial to note that Spiriva is available only through prescription, ensuring that it is used under appropriate medical supervision to manage chronic respiratory conditions effectively. The prescription-only status across all markets highlights the importance of healthcare provider guidance in its usage.
Conclusion
For those living with COPD or asthma, understanding the components, classification, and regulatory status of Spiriva is vital for effective treatment. As a prescription medication, it should be used under the supervision of healthcare professionals to ensure maximum benefit and safety in managing these chronic conditions.
Dosage & Administration of Spiriva
When managing conditions like COPD and asthma, understanding the right dosage of Spiriva is crucial. For COPD, the typical dosage is 18 mcg once daily via the HandiHaler. In cases of asthma, patients may use the Respimat inhaler at doses of either 2.5 mcg or 1.25 mcg depending on their specific needs.
It's essential to consider age and comorbidities when determining the appropriate dose. Elderly patients, as well as those with liver or kidney issues, should be carefully monitored to avoid potential complications. Always consult with a healthcare provider for personalized adjustments.
Treatment with Spiriva is intended for long-term maintenance. It is not designed for quick relief during an asthma attack or COPD exacerbation. Adherence to the prescribed regimen is key for ongoing symptom control. Missing doses may lead to a rebound effect, so it's advised to take Spiriva at the same time every day.
Storage is another important aspect to keep in mind. Spiriva capsules should be stored below 25°C in a dry environment. Moisture can affect efficacy, so it's vital to keep them protected. The Respimat device also has specific storage requirements, including keeping it capped when not in use and avoiding freezing.
Safety & Warnings for Spiriva
Before using Spiriva, certain contraindications must be considered. Known allergies to tiotropium, atropine, or any component of the medication can pose serious risks. Additionally, special caution is advised for patients with conditions like narrow-angle glaucoma and prostatic hyperplasia, as these could exacerbate side effects.
The potential side effects can range from common issues like dry mouth and sore throat to less common ones, such as hoarseness and sinusitis. Severe side effects, although rare, may occur and require medical attention. Being aware of how your body responds can aid in managing these effects.
Pregnant or breastfeeding women should consult their doctor before starting Spiriva. Individuals with liver or kidney impairments should also discuss usage with their healthcare provider to ensure safety. It’s vital to stay informed of any black box warnings associated with Spiriva, as they highlight serious safety concerns.
Patient Experience with Spiriva
User reviews from platforms like Drugs.com and Reddit reveal a mixed bag of experiences. Many patients report that Spiriva effectively manages their COPD and asthma symptoms. However, side effects such as dry mouth and occasional headaches are common concerns.
Insights from forums, including Facebook groups, reflect a community willing to share their thoughts. Common topics include challenges with adherence to the medication and discussions around reasons for discontinuation. Many users highlight the importance of consistency in taking Spiriva for optimal benefit.
When it comes to patient satisfaction, many users appreciate the long-lasting effects of Spiriva. However, some report a struggle with side effects leading to discontinuation or switching to alternative treatments. Understanding these perspectives can help inform treatment discussions.
Alternatives & Comparison to Spiriva
In the Canadian market, alternatives to Spiriva include Aclidinium, Glycopyrronium, and Umeclidinium. Each of these options has its unique benefits and considerations, making it essential for patients to explore all their choices.
| Alternative | Price | Effectiveness | Safety |
|---|---|---|---|
| Aclidinium | Similar to Spiriva | Effective for COPD | Generally safe |
| Glycopyrronium | Comparable | Proven efficacy | Minimal side effects |
| Umeclidinium | Often higher | Well-tolerated | Safe |
Local prescribing patterns indicate that healthcare providers may have preferences based on patient feedback and individual responses to these alternatives. Each patient’s needs will dictate the best choice, making discussions with healthcare professionals critical.
Market Overview (Canada)
When looking to get Spiriva in Canada, it's good to know where to find it. Pharmacies like Catena and HelpNet make it accessible, backed by a strong distribution network. You'll often find Spiriva stocked in larger pharmacy chains too, ensuring that patients don't struggle to locate their prescribed medication.
Getting into pricing, Spiriva generally ranges between CAD 60 to CAD 100 for a month’s supply, depending on the pharmacy and the specific prescription. It's essential to compare these prices with alternatives like Aclidinium or Umeclidinium, as they might offer cost-effective options without sacrificing efficacy.
Regarding packaging, Spiriva is available in multiple forms. The HandiHaler contains 18 mcg inhalation powder capsules in a blister pack, while the Respimat offers inhalation solutions in varying doses—2.5 mcg and 1.25 mcg per actuation. This variety in delivery methods allows for tailored patient preferences and needs.
Demand patterns have shifted notably, especially during and post-COVID. Increased awareness around respiratory health has spurred chronic usage, with many patients seeking longer-term management for conditions like COPD and asthma. This trend highlights the importance of Spiriva as a reliable treatment option for ongoing respiratory issues.
Research & Trends
Recent meta-analyses and clinical trials from 2022 to 2025 have provided valuable insights into Spiriva's effectiveness. These studies further emphasize its beneficial role in maintaining lung function for patients with COPD and asthma, contributing to better overall quality of life.
New research is exploring broader applications for Spiriva beyond its typical use cases. Current investigations are examining its potential effects on other respiratory conditions, signaling an expanding horizon for this established medication.
Concerning patent status, Spiriva remains protected under existing patents, making generics unavailable in the Canadian market for now. However, patients should stay informed, as generics may become accessible in the near future once those patents expire, opening doors to more affordable treatment options.
Guidelines for Proper Use
Taking Spiriva correctly is crucial for its effectiveness. It’s best taken once daily, ideally at the same time to maintain consistency. While some may wonder about timing relative to meals, it can be taken with or without food, making it particularly easy to fit into a daily routine.
There are a few key points to keep in mind for optimal use:
- Avoid mixing Spiriva with other medications unless advised by a healthcare provider.
- While moderate alcohol consumption doesn’t typically interfere, consulting a doctor is always prudent.
- Store Spiriva in a cool, dry place below 25°C to ensure potency.
Common mistakes include double dosing if a dose is missed and improper inhalation techniques, which can affect how well the medication works. Reading the patient leaflet can clarify how to use it correctly and emphasizes the importance of discussing any uncertainties with a healthcare professional.
Following these guidelines is essential for anyone relying on Spiriva, helping to ensure effective management of respiratory conditions while avoiding complications.