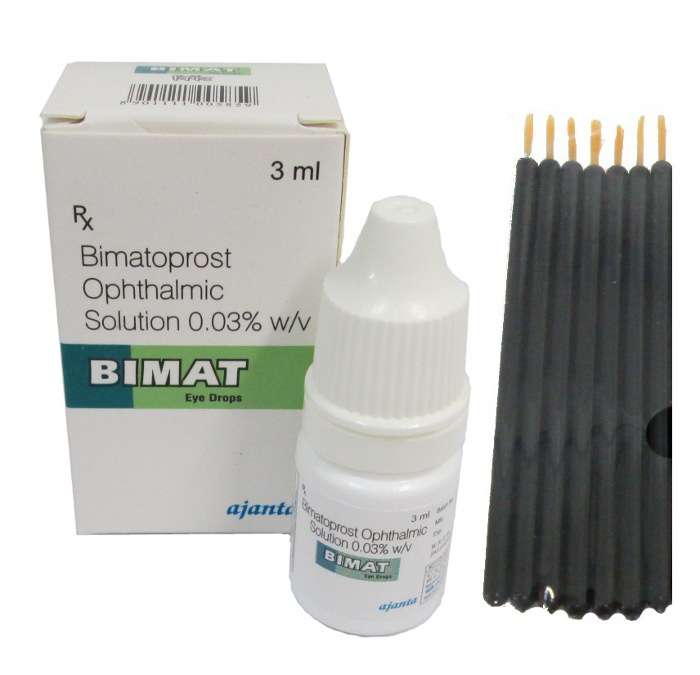
Lumigan + Applicators
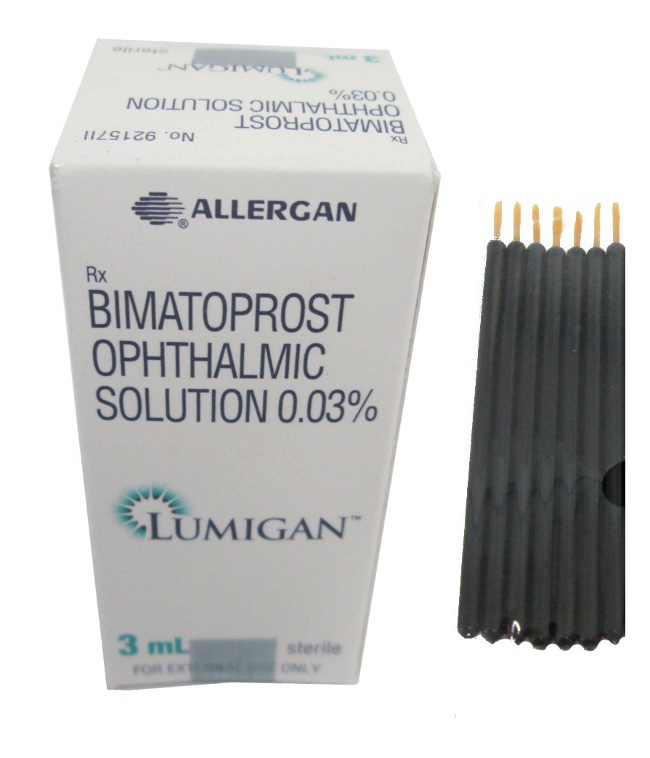
Lumigan + Applicators
- In our pharmacy, you can buy Lumigan + Applicators with a prescription, available through most major hospital and retail chains.
- Lumigan is used for the reduction of intraocular pressure in open-angle glaucoma and ocular hypertension. It works as a prostaglandin analog, increasing the outflow of aqueous humor.
- The usual dosage of Lumigan is 1 drop in the affected eye(s) once daily in the evening.
- The form of administration is an ophthalmic solution.
- The effect of the medication typically begins within 4 to 6 hours.
- The duration of action is 24 hours.
- Limit alcohol consumption while using Lumigan.
- The most common side effect is conjunctival hyperemia (red eyes), occurring in approximately 31% of users.
- Would you like to try Lumigan + Applicators without a prescription?
Basic Lumigan + Applicators Information
- INN (International Nonproprietary Name)
- Brand names available in Canada
- ATC Code
- Forms & dosages (e.g., tablets, injections, creams)
- Manufacturers in Canada
- Registration status in Canada
- OTC / Rx classification
International Nonproprietary Name (Inn)
Bimatoprost is the International Nonproprietary Name (INN) for a medication primarily used to lower intraocular pressure in patients with open-angle glaucoma. As a prostaglandin analog, it operates by enhancing the outflow of aqueous humor, thus effectively managing the condition.
Brand Names in Canada
In Canada, Bimatoprost is marketed under several brand names, including:
- **Lumigan**
- **Latisse**
- **Lumigan RC**
Additional terms often encountered include "Lumigan Eye Drops" and "Bimatoprost Drops," categorized by concentration, such as "Lumigan 0.01%" and "Lumigan 0.03%." This wide array of terminology helps distinguish between various products and their specific applications.
ATC Code
The Anatomical Therapeutic Chemical (ATC) classification code for Bimatoprost is S01EE03. This code is crucial as it helps categorize the medication within the Sensory Organs category, under the Ophthalmologicals and Prostaglandin analogues subset.
Forms & Dosages Available
Bimatoprost is available in different concentrations and packaging formats. It is offered as an ophthalmic solution in bottles containing:
| Concentration | Form | Packaging |
|---|---|---|
| 0.01% (0.1 mg/mL) | Ophthalmic solution | 2.5 mL, 3 mL, 5 mL bottles |
| 0.03% (0.3 mg/mL) | Ophthalmic solution | 2.5 mL, 3 mL, 5 mL bottles |
Standard **Lumigan** packaging typically includes a white dropper, allowing for controlled dispensing of the drops.
Manufacturers and Registration Status
The primary manufacturer of Bimatoprost in Canada is Allergan, which is a subsidiary of AbbVie. As the original manufacturer, Allergan holds the registration for this product, ensuring compliance with Canadian regulations and standards.
Classification
Bimatoprost is classified as a prescription-only medication (Rx). It is not available over-the-counter (OTC) in Canada. This classification is essential, as it informs both patients and healthcare providers about the necessity of medical oversight when using this medication.
Mechanism of Action of Lumigan
Ever wonder how Lumigan helps those with eye pressure issues? Simply put, it increases fluid drainage from the eye, reducing the pressure that can lead to serious conditions like glaucoma.
In clinical terms, Lumigan is a prostaglandin analog that specifically binds to FP receptors. This action enhances the outflow of aqueous humor, the fluid in the eye, which plays a key role in maintaining eye pressure. The effectiveness of Lumigan comes from this unique interaction, ensuring that the eye remains healthy while minimizing the risks associated with elevated intraocular pressure.
Onset, Metabolism, and Elimination of Lumigan
When using Lumigan, noticeable effects generally kick in within just 1 to 2 hours after application. This quick onset provides relief for those struggling with eye pressure and different forms of glaucoma.
How does the body handle Lumigan once it's in? It's metabolized primarily in the liver, breaking down into various compounds before elimination. The half-life of Lumigan in the eye is approximately 45 minutes, indicating how quickly the body processes the medication. These aspects are crucial for understanding the pharmacokinetics of Lumigan, allowing patients and healthcare providers to plan effective treatment regimens.
Interactions with Lumigan
<pThere’s always a concern about how different medications work together — and Lumigan is no exception. It can have potential interactions with systemic medications, especially those that impact ocular drainage. This is something every patient should consider when adding new prescriptions to their routine.- Common Drug Interactions: Medications that lower blood pressure or other ocular hypotensive agents can interact with Lumigan.
- Food Interactions: Generally, no specific food interactions are noted; however, always consult with a healthcare provider for personalized advice.
- Effects of Alcohol: Moderate alcohol intake is usually safe, but excessive consumption might lead to unwanted side effects or complications.
Indications for Lumigan and Applicators
Why might someone consider using Lumigan? Well, the primary reason is to manage conditions that elevate intraocular pressure. It’s a well-accepted treatment for open-angle glaucoma and ocular hypertension. Glaucoma, often sneaky and silent, can lead to vision loss if untreated. By using Lumigan, patients aim to maintain eye health and improve their quality of life.
Many might be surprised to discover that Lumigan isn't just for treating glaucoma. Off-label, it’s become increasingly popular for enhancing eyelash growth when used with applicators like those found in Latisse packaging. Cosmetic applications are gaining traction in Canada, underlining a shift in how bimatoprost is perceived and utilized.
When considering Lumigan for different populations, special care is paramount. Pediatric use is generally off-label, leading to consultations with healthcare professionals. It's crucial to monitor elderly patients more closely for side effects. And for pregnant individuals, assessing the risks and benefits is essential before starting treatment, ensuring unmatched safety in delicate situations.
Dosage & Administration of Lumigan
What’s the usual scoop on dosing? The typical regimen for administering Lumigan is pretty straightforward: instill 1 drop in the affected eye(s) once daily in the evening. It’s important to adhere to this schedule for optimal efficacy, as straying from it can diminish the drug's intended benefits.
Monitoring is particularly advised for elderly patients, but generally, no significant adjustments are needed for them. For those with liver or kidney concerns, some guidelines suggest caution. It is wise to regularly review treatment plans with healthcare providers to ensure safety and effectiveness.
As for storing your Lumigan drops? Keep them between 15-25°C (59-77°F) and away from light. Proper storage is crucial for maintaining the potency of these eye drops and ensuring you get the best results when applying them.
Safety & Warnings for Lumigan
Let’s be clear about safety: Lumigan has critical contraindications. Absolute contraindications include hypersensitivity to bimatoprost, meaning if you've had an allergy to it before, it should be avoided. Furthermore, it’s not approved for children under three years.
For some individuals, relative contraindications might come into play. Patients with inflammatory eye conditions need monitoring during treatment, as should those with risk factors for intraocular infections.
Side effects can vary. Mild ones may include conjunctival hyperemia, itching, or dryness. On the other hand, severe side effects may involve changes in iris pigmentation or eyelid skin texture. No black box warnings are presently labeled, but it's important to be cautious about potential risks, especially when dealing with infections.
Keeping tabs on these details can help manage expectations and foster better communication with healthcare providers. Remember, awareness is key for making informed decisions regarding treatment.